SAIFx Technology
An Innovation in Nutrient Delivery
Liposomes are tiny phospholipid bubbles with a bilayer structure very similar to that of our cell membranes. In the 1960’s, scientists discovered the use of liposomal delivery in medicine. By encapsulating a drug within a phospholipid, the drug became highly biocompatible, delivering measured amounts of therapeutic substances to specific tissues of the body.
Liposomes are able to carry both fat and water-soluble payloads, making them ideal for nutrient bioavailability.
A very small number of nutritional supplement manufacturers are currently pioneering the benefits of this unique science within the nutraceutical industry.
GMPriority Pharma are at the forefront of this evolution.
GMPriority Pharma have been involved in the research and development of liposomal encapsulation for over 15 years and made our products commercially available in 2012.
SAIFx® technology represents a quality by design approach, implementing innovation and quality throughout all aspects of the development and manufacturing processes.
This results in proprietary, stable and efficient formulations of authenticated liposomal content.
SAIFx® Technology
SCIENTIFIC (History)
GMPriority Pharma’s foundation is its robust scientific approach.
The company has over 35 years of combined expertise in liposome technology, leveraging nanoencapsulation for enhanced delivery and absorption of nutrients. The SAIFx® process ensures that the liposomes are meticulously engineered for precise size, stability, and efficacy.
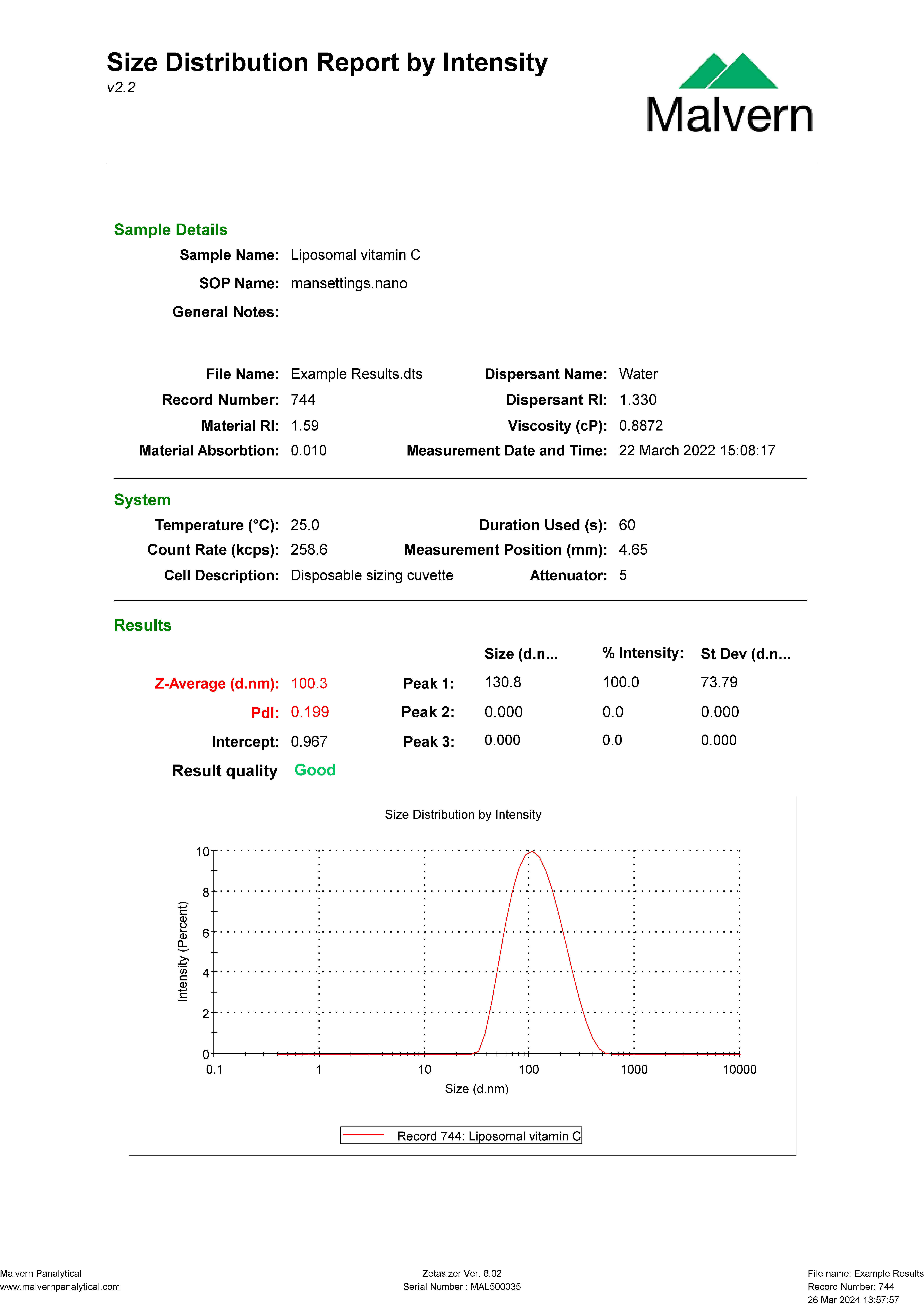
Independent peer-reviewed studies provide tangible evidence of the superior bioavailability achieved with GMPriority Pharma’s liposomal formulations. For instance, our liposomal vitamin C demonstrated exceptional plasma levels compared to standard formulations, as shown in comparative studies.
Additionally, we collaborate with academic institutions such as Anglia Ruskin University to validate and refine our liposomal technologies. This partnership continues to yield ground-breaking innovations, including refined liquid liposomal formulations and dry liposomal powders, a world first in the nutraceutical field. SAIFx® technology is globally recognised as the gold standard for nutraceutical liposomal formulation.

AUTHENTIC (Characterisation)
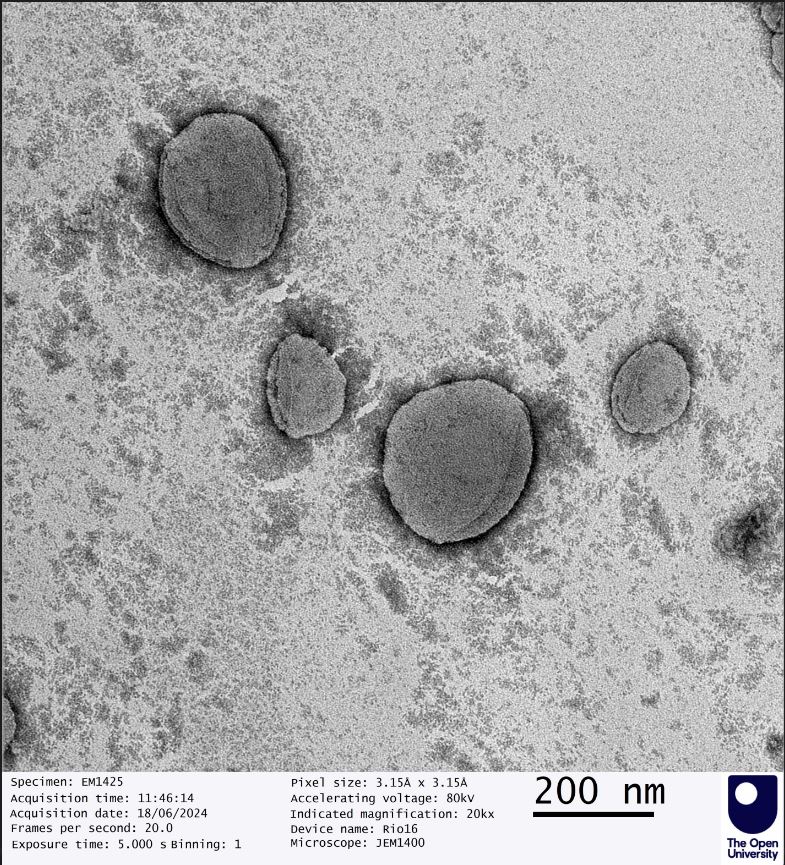
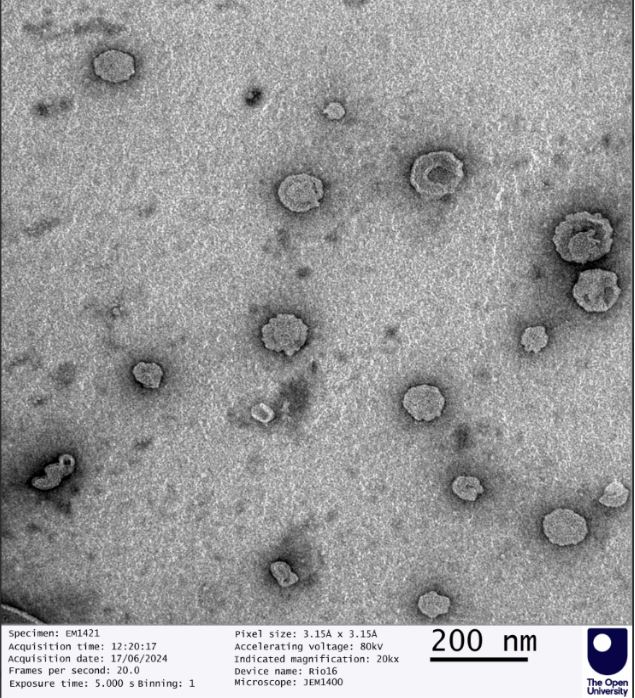
Authenticity is a cornerstone of GMPriority Pharma. Our commitment to transparency and rigorous testing ensures all formulations meet the highest industry standards. Every batch undergoes advanced quality control measures, including Transmission Electron Microscopy (TEM), to verify particle size, distribution, and structural integrity.
The authenticity extends to ingredient sourcing, prioritising biocompatibility and biodegradability. This guarantees liposomal products are not only effective but also environmentally conscious and safe for consumption.
A testament to our dedication is the ever increasing library of third-party validations our products have received. These include clinical trials and scholarly articles, published in peer-reviewed journals, solidifying GMPriority Pharma’s reputation as a leader in liposomal science.
The GMPriority Pharma R&D division also supports custom projects, offering services such as feasibility studies, formulation development, stability testing and analytical characterisation. The company’s focus on scaling up establishes a seamless transition from concept to GMP-compliant production, supporting regulatory requirements.
INNOVATIVE (Methodology)
The driving force behind GMPriority Pharma’s success is innovation. The development of SAIFx® technology represents a significant leap forward in liposomal encapsulation science. Our purpose-built, state-of-the-art UK manufacturing facilities, incorporating hi-tec R&D laboratories, Class 7 cleanroom and accredited FSSC ISO 22000 production suite results in the creation of smaller, single-layer liposomes with unparalleled stability and efficiency.
Working within the interface between industry and academia, Professor Najlah as the Chief Scientist and Co-Founder of the company is able to assimilate, then transfer knowledge between sectors to valuable effect. In particular, his research on developing scaling-up technologies for nanomedicines has attracted several collaborations with industries, which led to KTP and KEEP+ projects funded by Innovate UK and European Regional Development Fund, respectively.
The innovations are not limited to liquid formulations. The company’s dry liposomal powders, developed through a three year collaborative project supported by Innovate UK, mark another revolutionary step forward. These powders provide the same superior bioavailability and stability as the liquid formulas, but offer versatility for multiple applications.
GMPriority Pharma’s packaging solutions also reflect our innovative spirit. Options such as Unicadose®, compact and pre-measured, provide consistent dosing and convenience for consumers.

FORMULATIONS
Meticulously crafted, stable formulations are at the heart of what drives GMPriority Pharma.
Using our proprietary SAIFx® technology, the liposomes encapsulate bioactive compounds in high quality, single-layer spheres designed to maximise bioavailability.
The formulations are tailored to deliver nutrients into systemic circulation, bypassing traditional absorption barriers.
The SAIFx® product development process mandates rigorous stability studies, advanced characterisation, and continuous quality improvement from laboratory to full production scale. Each formulation’s design begins with a detailed understanding of the source, benefits and consistency of individual ingredients.
From liquid supplements to dry powders, GMPriority Pharma offers a diverse range of products designed to meet the unique needs of all clients. These formulations are utilised by leading pharmaceutical and nutraceutical brands worldwide, solidifying the company’s position as a global leader in liposomal innovation.
SAIFx® Technology: A Vision for the Future
SAIFx® encapsulates GMPriority Pharma’s philosophy: merging science, authenticity, innovation and premium formulations to deliver unparalleled quality. This technology not only enhances the bioavailability and efficacy of liposomal supplements but also sets new benchmarks for the nutraceutical industry.
As the global market for liposomal supplements continues to grow, GMPriority Pharma remains at the forefront, shaping the future of bioavailability in nutraceutical science. Our dedication to advancing liposomal technology ensures that consumers receive products that are scientifically validated, authentically produced, innovatively designed, and expertly formulated.
By choosing GMPriority Pharma, clients and consumers alike benefit from a legacy of excellence
and a commitment to health and well-being.

SAIFx® Technology - Evidence File
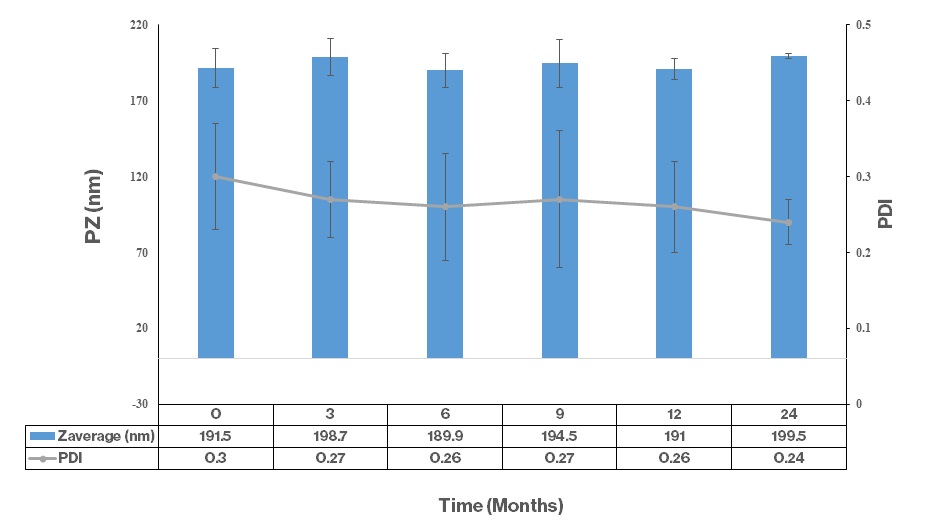
Particle Size Average and PDI of Vitamin C – 24 Months
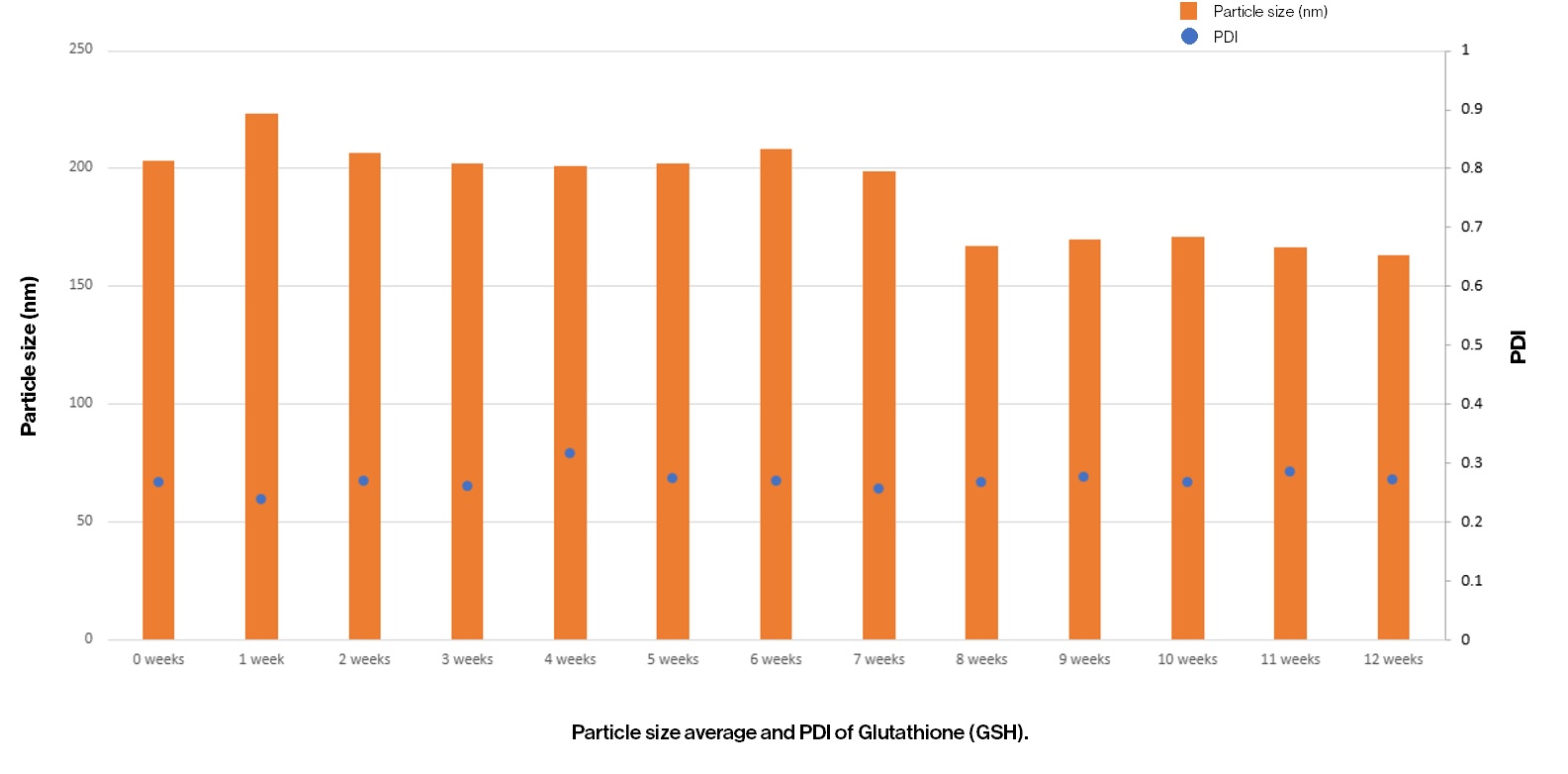 Particle size average and PDI of Glutathione (GSH) – 12 weeks.
Particle size average and PDI of Glutathione (GSH) – 12 weeks.
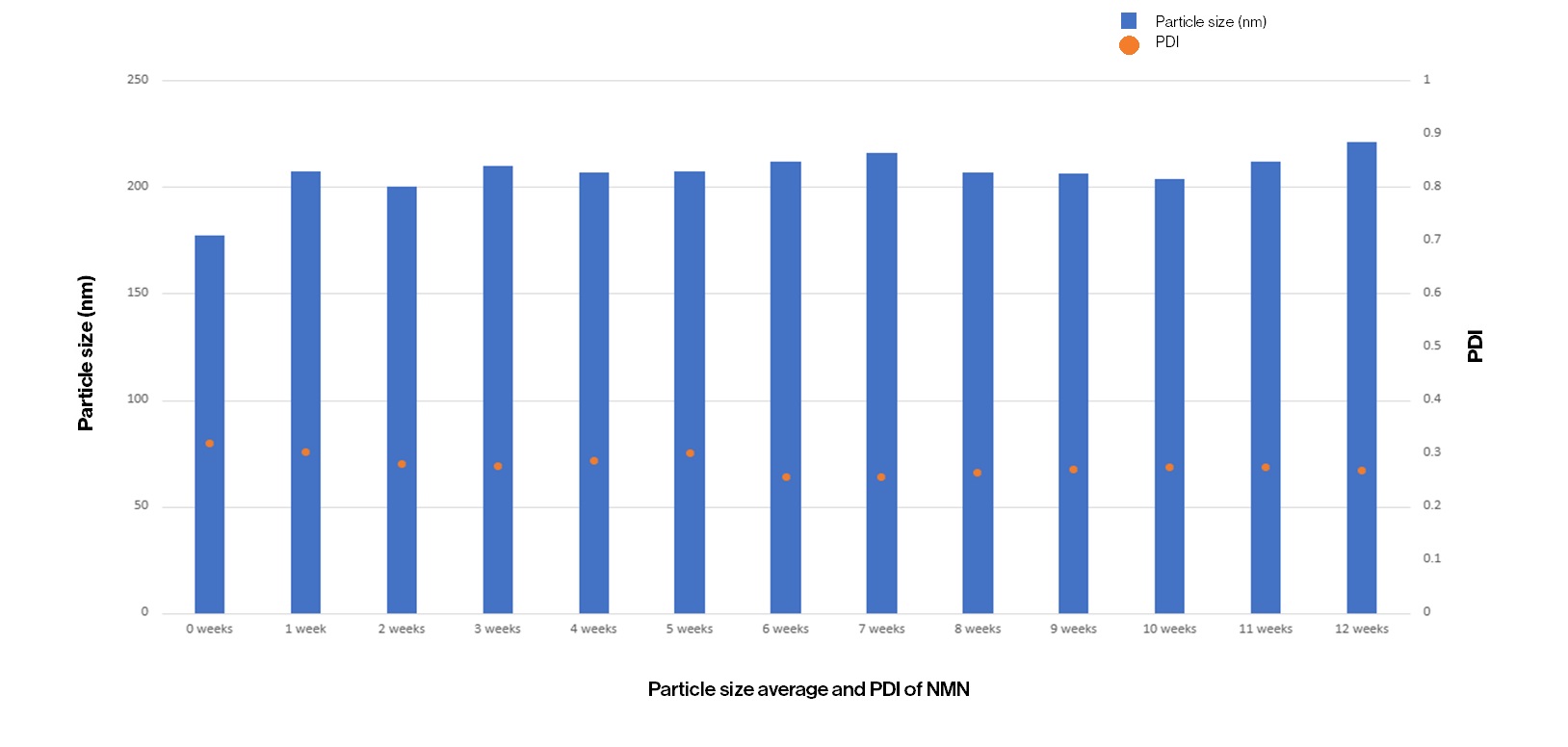
Particle Size Average and PDI of NMN
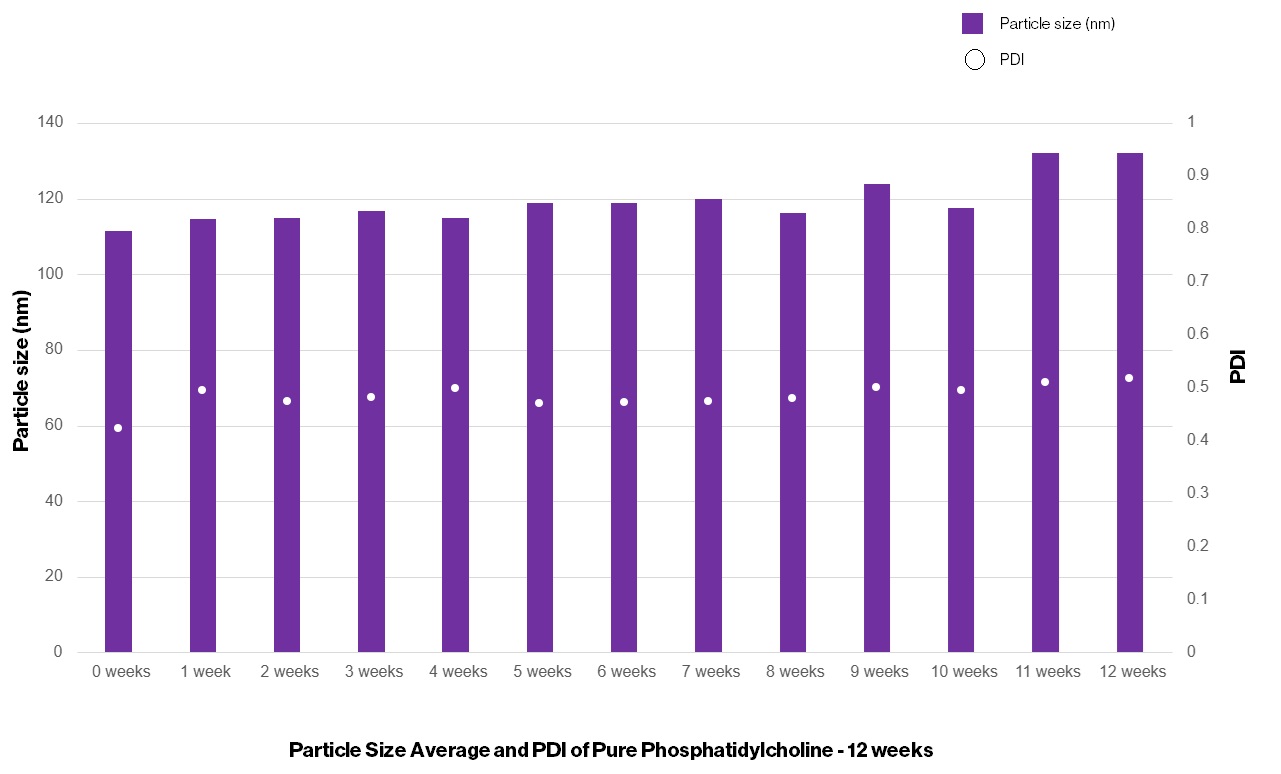
Particle Size Average and PDI of Pure PC – 12 weeks
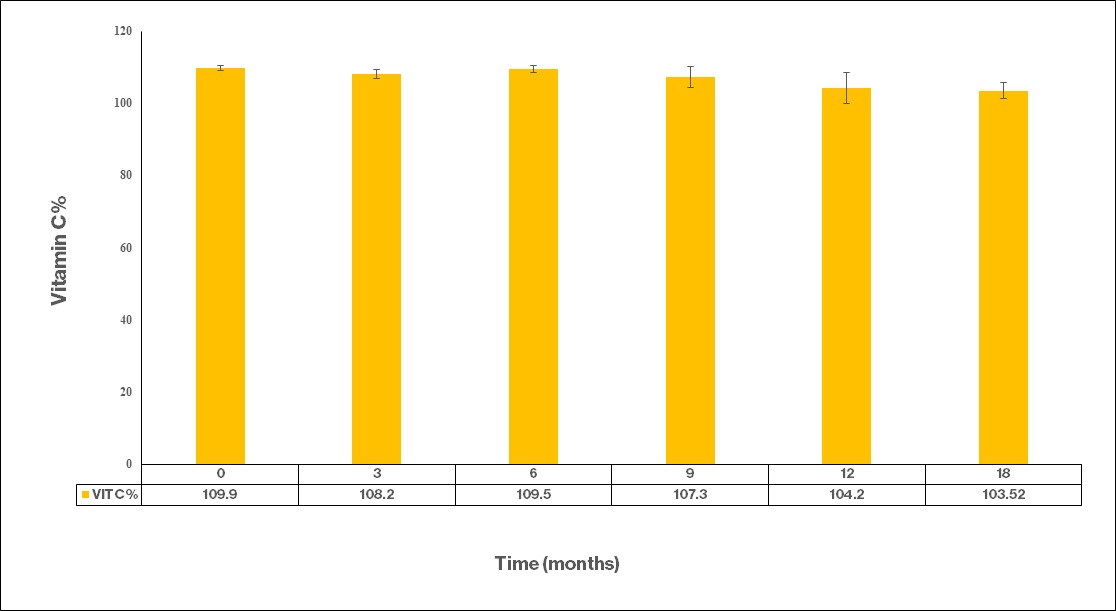
Average Vitamin C Concentration – 18 months
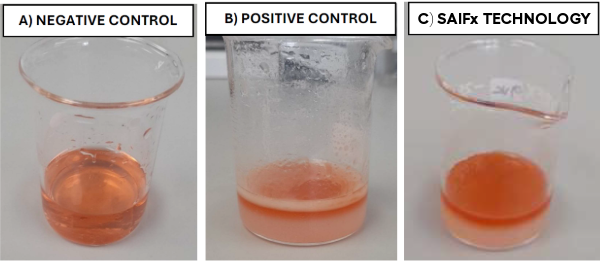
Example of SAIFx® Technology under Sudan Test characterisation

 ]
]