GMPriority Pharma Unveils Groundbreaking Innovation in Liposomal Dry Powder Technology with Successful Scale-Up of dry SAIFx®

GMPriority Pharma is thrilled to announce the successful scale-up of our pioneering liposomal dry powder technology, dry SAIFx®, following a highly productive two-year Research and Development Knowledge Transfer Partnership (KTP) with Anglia Ruskin University. This innovative advancement marks a significant milestone in liposomal encapsulation technology, showcasing our commitment to scientific excellence and quality.
Revolutionising Liposomal Delivery with dry SAIFx®
The development of dry SAIFx® represents a major leap forward in liposomal drug delivery systems. Traditional liposomal formulations have predominantly been liquid-based, which poses challenges in terms of stability, shelf-life, and transportation. The dry SAIFx® technology addresses these issues by converting liposomal formulations into a dry powder form that can be easily reconstituted without compromising the integrity or efficacy of the liposomes.
Unrivalled Quality and Performance
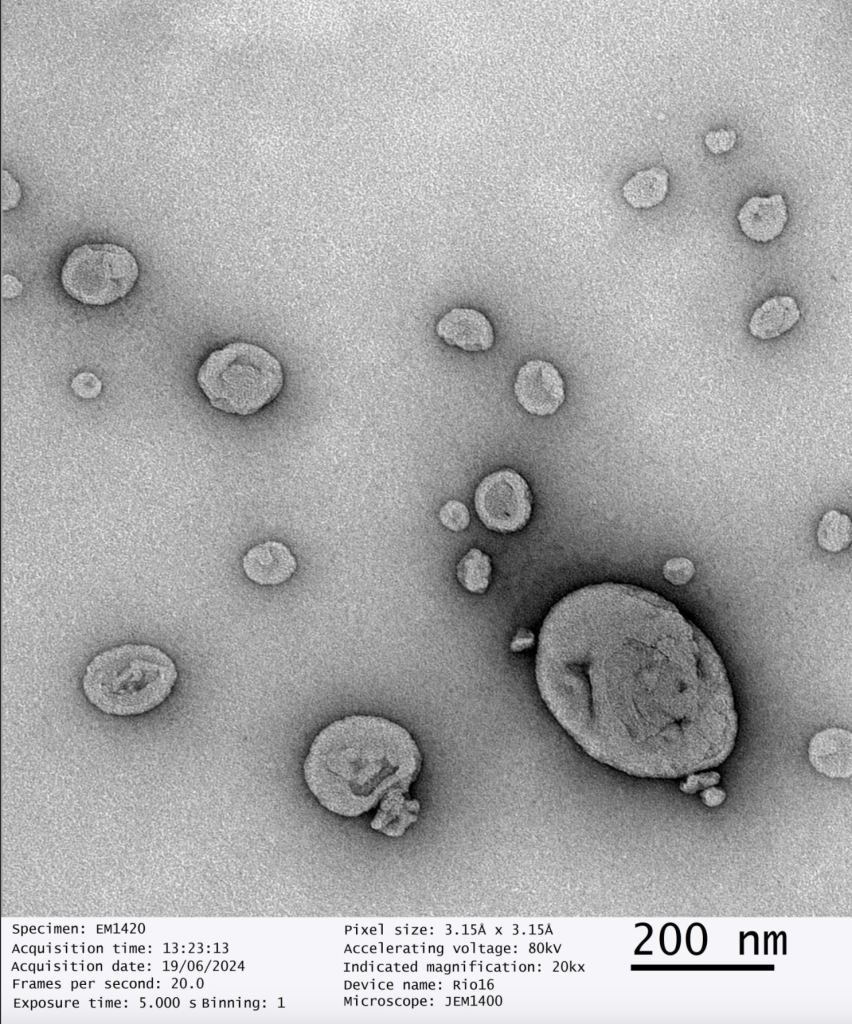
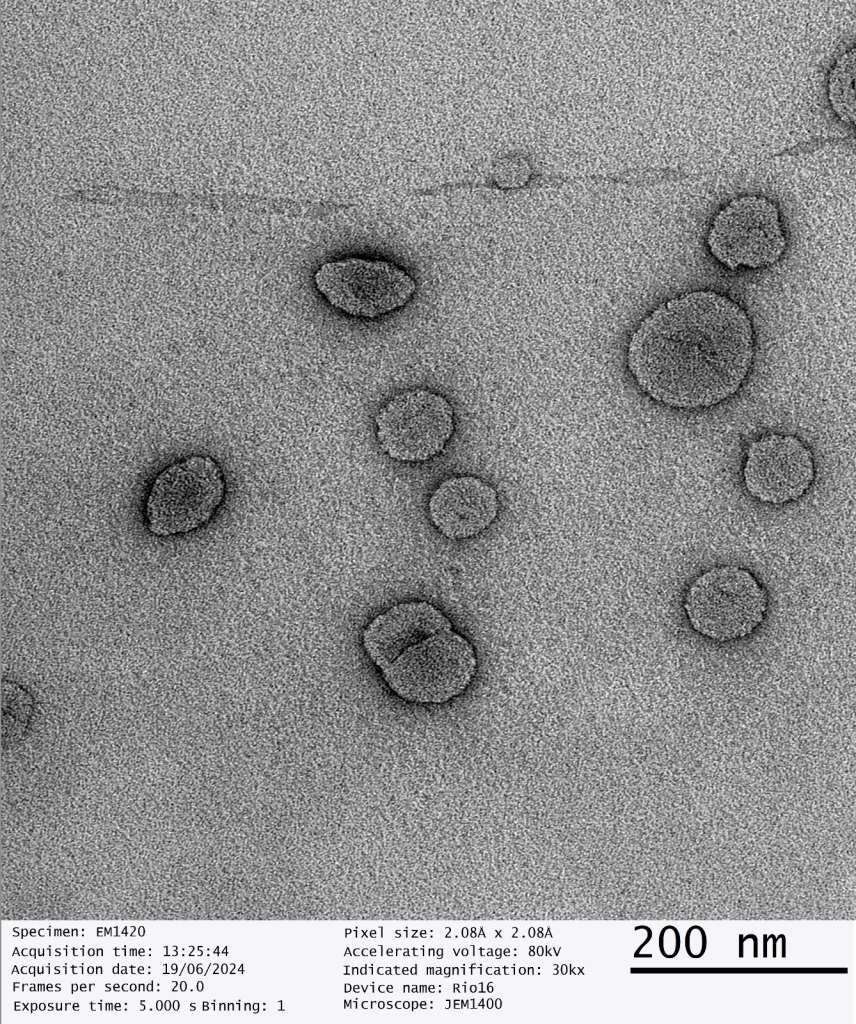
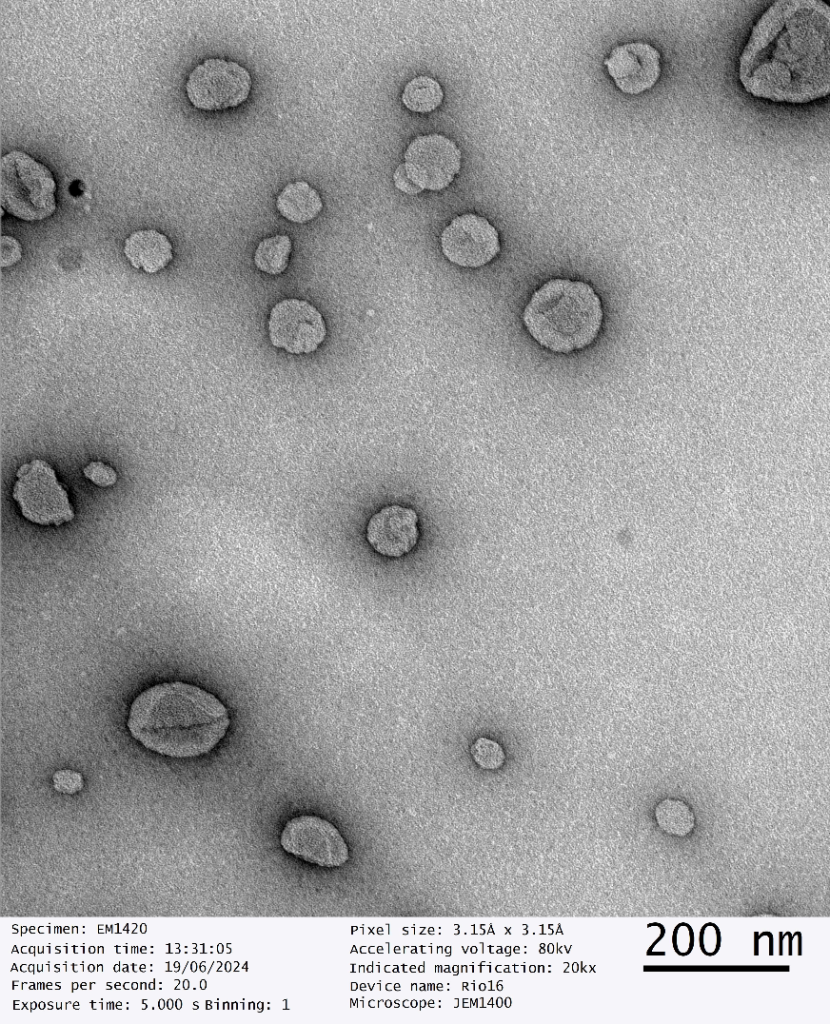
Our dry SAIFx® technology stands out due to its exceptional quality, as confirmed by third party, advanced Transmission Electron Microscope (TEM) imaging. The TEM images of our reconstituted dry powders reveal perfectly intact liposomes, demonstrating the robustness and reliability of our drying process.
This breakthrough ensures that the therapeutic efficacy and bioavailability of the liposomal products are maintained, providing superior performance compared to conventional methods.
Professor Najlah, Chief Scientist at GMPriority Pharma, commented:
“The successful scale-up of dry SAIFx® is a testament to our team’s dedication and expertise. The TEM imaging results speak volumes about the quality and stability of our reconstituted liposomes, positioning GMPriority Pharma at the forefront of wet and dry liposomal encapsulation technology.”
Collaboration with Anglia Ruskin University
The two-year R&D KTP project with Anglia Ruskin University played a crucial role in achieving this innovation. The collaboration brought together the best minds in the field, combining academic knowledge with industrial expertise to overcome the technical challenges associated with liposomal dry powder technology.
Acknowledging Our Team of Scientists
This achievement would not have been possible without the relentless efforts and dedication of our incredible team of scientists. Their expertise and innovative thinking have been instrumental in overcoming the challenges associated with scaling up the dry SAIFx® technology. We extend our deepest gratitude to everyone involved in this remarkable project.
Future Directions and Impact
The successful development and scale-up of dry SAIFx® open new avenues for liposomal product applications across various industries, including pharmaceuticals, nutraceuticals, and cosmetics. By providing a stable, easy-to-transport, and highly effective liposomal delivery system, GMPriority Pharma continues to lead the way in innovative solutions that enhance the bioavailability and efficacy of active compounds.