Dry Liposomal Powder
True Innovation
The result of a three-year, Innovate UK collaborative project with Anglia Ruskin University.
By implementing our innovative SAIFx® technology, we have successfully achieved scaling up of the world's first true dry liposomes for nutraceutical application.

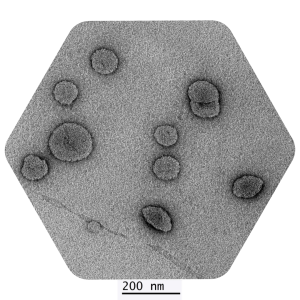
Third party validated, our dry liposomal powders have been scrutinised under Transmission Electron Microscopy which determines the size, shape and distribution of nanoparticles.
Dry Powder Liposomal Glutathione Analysis
Date of Analysis 19th June 2024
Microscope JEM1400
Magnification 30kx
The Benefits of Dry Liposomal Powder
Enhanced stability
Ease of storage and transportation
Improved formulation flexibility
Enhanced bioavailability
Palatability
Convenience
The development of dry liposome powder represents a significant innovation in the field of drug delivery and nutritional supplements. This technology offers numerous potential uses and advantages over traditional liquid liposomal formulations, enhancing the efficacy, stability, and convenience of liposomal products.
Potential Uses of Dry Liposome Powder
Pharmaceutical Applications:
- Drug Delivery: Dry liposome powders can be used to encapsulate a variety of drugs, improving their bioavailability and targeting specific tissues or cells within the body.
- Vaccines: Encapsulating vaccines in liposomal powders can enhance their stability and immune response, providing a more effective delivery method.
- Antibiotics: Liposomal encapsulation can help in the controlled release of antibiotics, reducing the required dosage and minimising side effects.
Nutritional Supplements:
- Vitamins and Minerals: Dry liposome powders can encapsulate vitamins and minerals, significantly improving their absorption rates and bioavailability compared to traditional supplements.
- Antioxidants: Compounds like curcumin, resveratrol, and glutathione can be encapsulated in liposomes to enhance their stability, palatability and efficacy.
- Amino Acids and Peptides: Encapsulating amino acids and peptides can protect them from degradation in the gastrointestinal tract, ensuring better absorption and utilisation.
Cosmetic and Skincare Products:
- Anti-Aging Formulations: Liposomal encapsulation of active ingredients like retinoids, hyaluronic acid, and peptides can enhance their penetration into the skin and prolong their activity.
- Moisturisers and Serums: Encapsulated liposomal ingredients can improve the delivery and efficacy of moisturisers and serums, leading to better skin hydration and nourishment.
Veterinary Medicine:
- Animal Health Supplements: Liposomal powders can be used to deliver nutrients and medications to pets and livestock, improving their health and reducing the frequency of dosing.
- Vaccines for Animals: Encapsulation of veterinary vaccines can enhance their stability and immune response, providing more effective protection for animals.
Advantages of Dry Liposome Powder
Enhanced Stability:
- Dry liposome powders exhibit significantly greater stability compared to liquid formulations. Removing water from the liposomes prevents degradation and hydrolysis, extending the shelf life of the product.
Improved Bioavailability:
- The encapsulation of active ingredients in liposomes improves their bioavailability, ensuring more efficient absorption and utilisation by the body. This is particularly important for compounds with poor solubility or stability.
Ease of Storage and Transportation:
- Dry powders are easier to store and transport than liquid formulations. They do not require refrigeration and are less prone to leakage or spoilage, making them more convenient for distribution, export and use in various environments.
Controlled Release:
- Liposomal encapsulation allows for the controlled release of active ingredients, providing a sustained therapeutic effect and reducing the frequency of dosing. This can enhance patient compliance and treatment efficacy.
Versatility in Formulation:
- Dry liposome powders can be easily reconstituted in water or other solvents before use, providing flexibility in formulation and application. They can be incorporated into various dosage forms, including capsules, tablets, and topical creams.
Reduction of Side Effects:
- By targeting the delivery of active ingredients to specific tissues or cells, liposomal encapsulation can reduce systemic exposure and minimise potential side effects, improving the safety profile of the product.
Enhanced Solubility of Hydrophobic Compounds:
- Liposomes can encapsulate hydrophobic (water-insoluble) compounds, improving their solubility and bioavailability. This enables the use of compounds that would otherwise be difficult to formulate and administer effectively.
Protection from Degradation:
- Encapsulation in liposomes protects active ingredients from degradation by enzymes, pH, and other environmental factors, ensuring that they remain effective until they reach their target site.
Dry liposome powder technology offers numerous advantages over traditional formulations, including enhanced stability, improved bioavailability, and ease of storage and transportation. These benefits make it a versatile and powerful tool for delivering pharmaceuticals, nutritional supplements, and cosmetic ingredients more effectively.
As GMPriority Pharma continues to innovate and expand the applications of this technology, the potential for improving health outcomes and product performance grows exponentially.
Independent Third Party Validation of GMPriority Pharma Dry Powder Liposomes
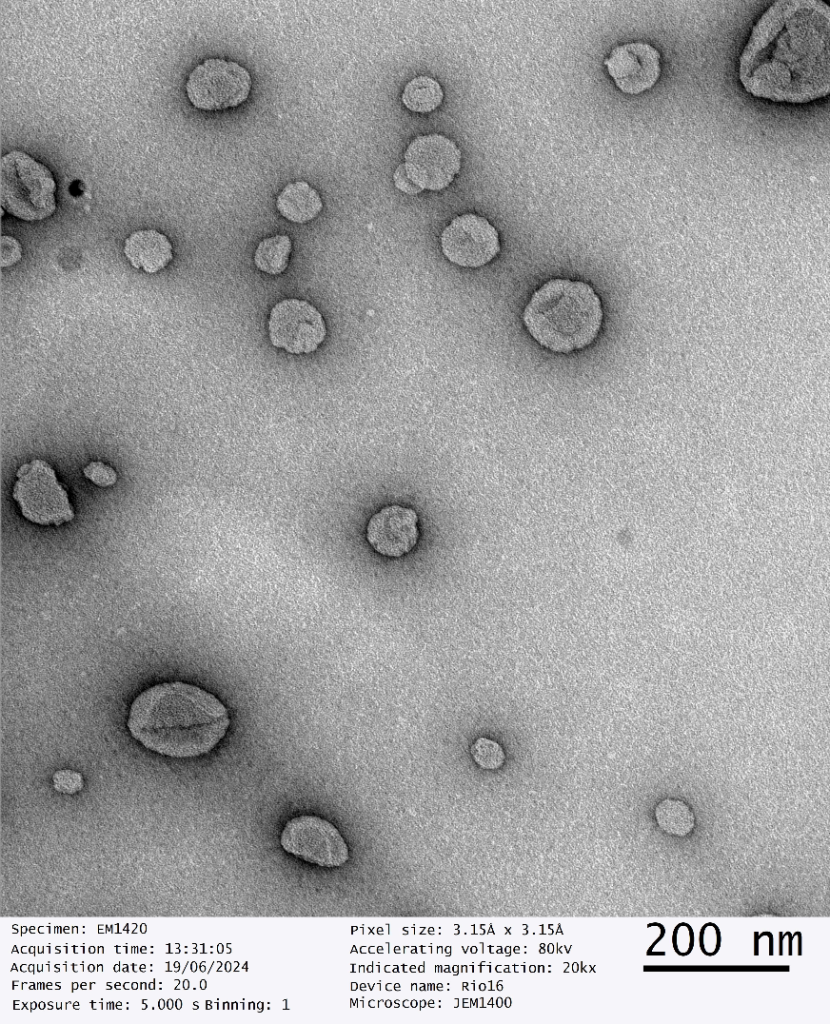
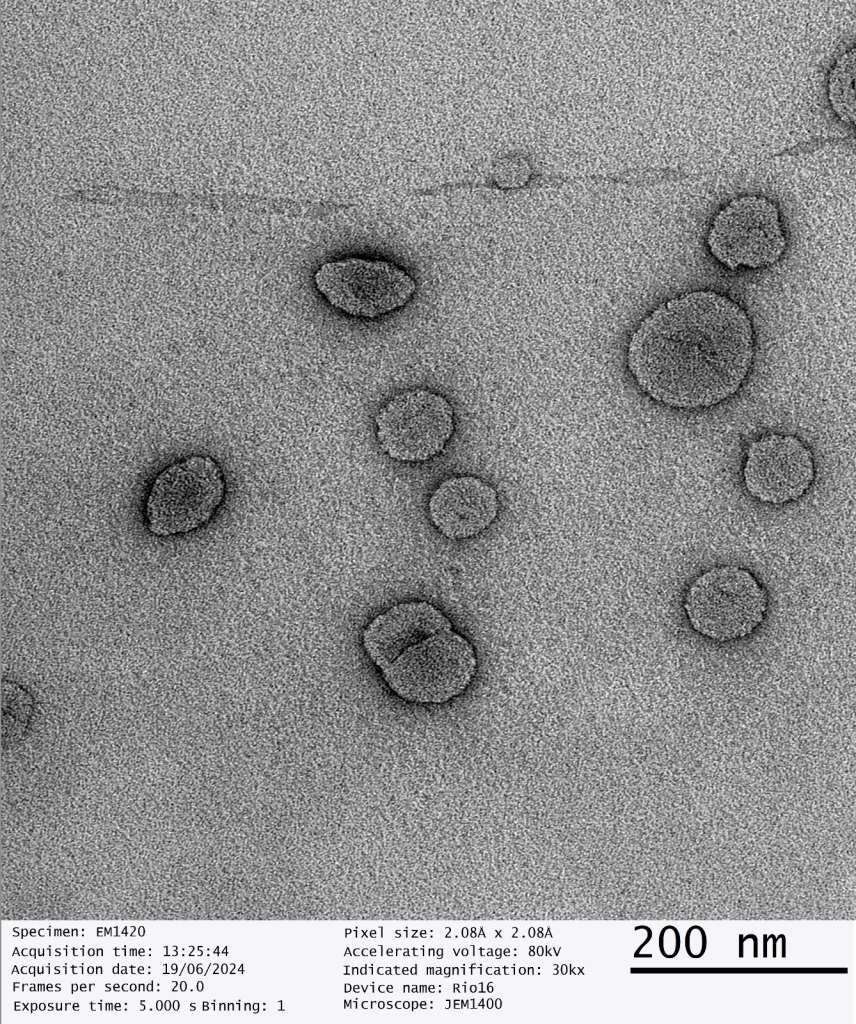
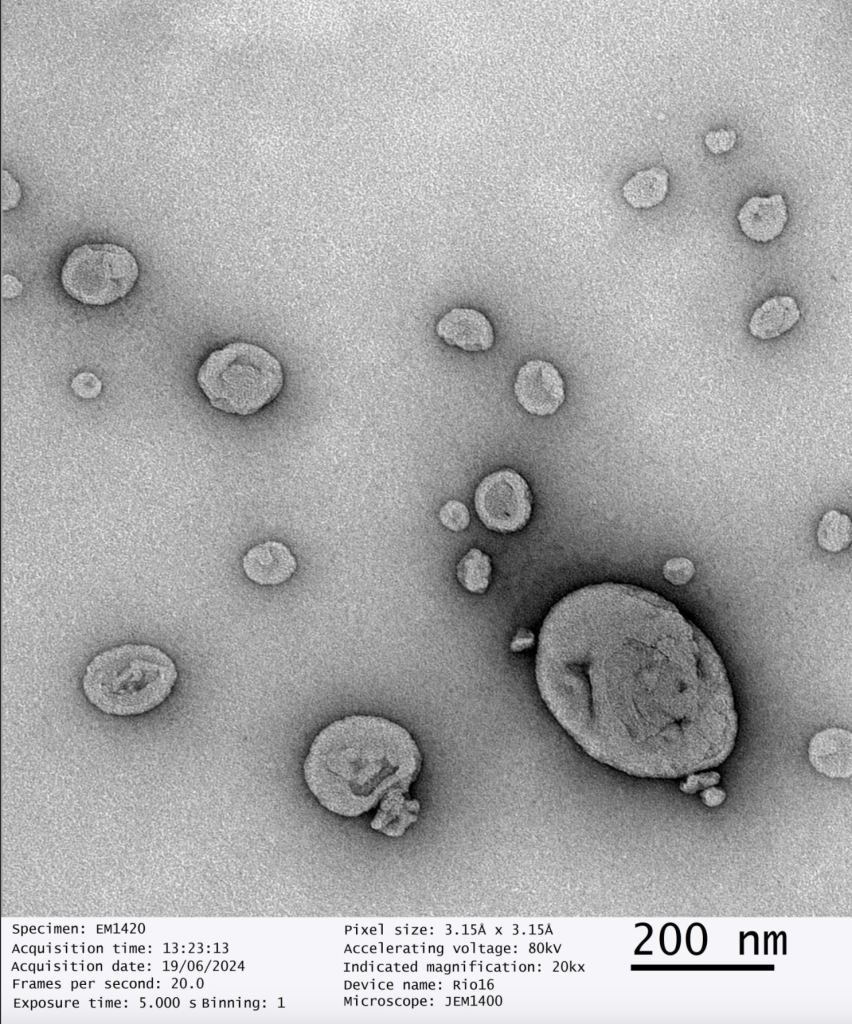
GMPriority Pharma Dry Liposome Powder R&D
GMPriority Pharma Manufacturing Methods for Dry Powder Liposomes
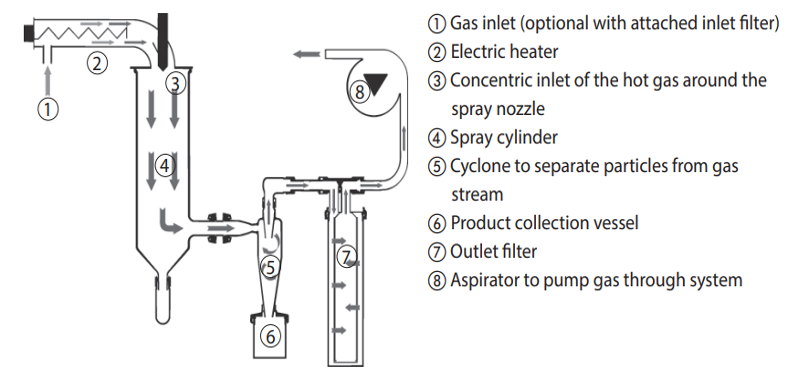
Spray Drying
Spray drying is a common manufacturing method for producing dry powder liposomes. Spray drying is a simple, robust, continuous process.
In this process, a liquid liposomal suspension is atomised into fine droplets, which are then rapidly dried using hot air or inert gases. The resulting powder consists of individual or aggregated liposomes, encapsulating the active ingredient.
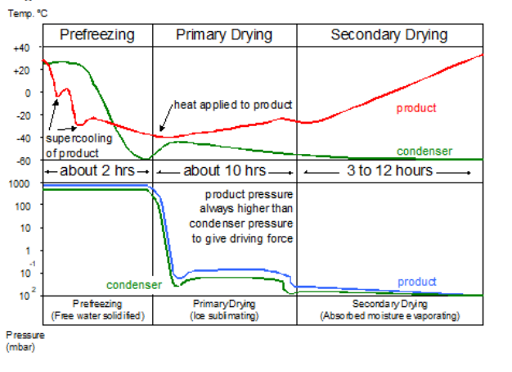
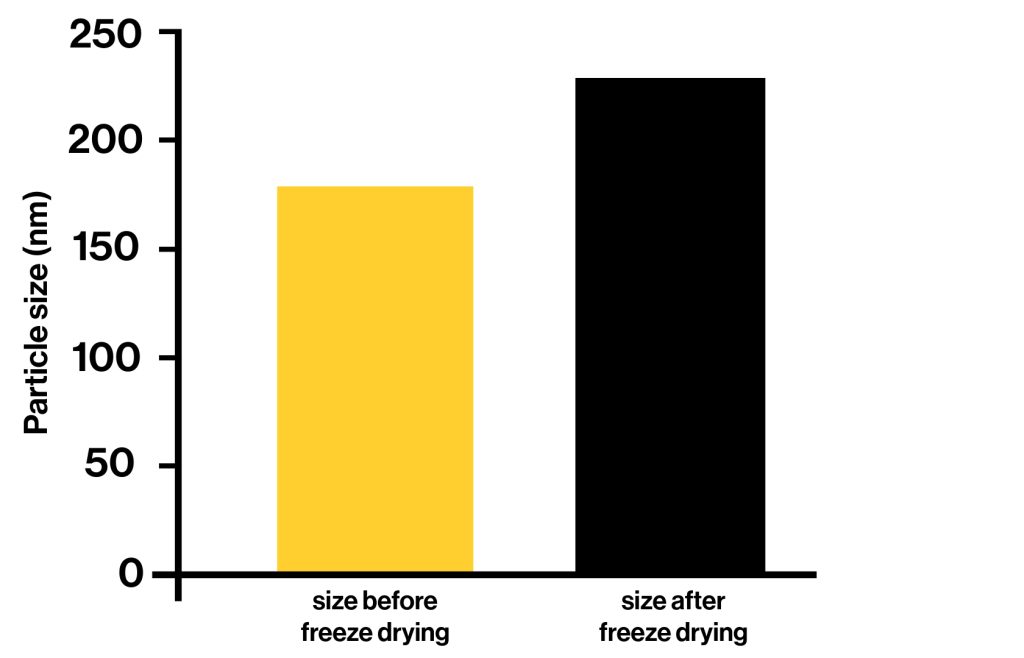
Freeze Drying (Lyophilisation)
Freeze drying, also known as lyophilisation, is the process of freezing a liquid liposomal suspension followed by sublimation of ice under vacuum conditions.
Essentially, freeze drying is a dehydration technique. What differentiates the freeze drying process from other dehydration techniques, is that dehydration takes place whilst the product is in a frozen state and in a vacuum.
This gentle drying process preserves the integrity of the liposomal structure and prevents aggregation, resulting in a dry powder with high encapsulation efficiency and stability.
Both spray drying and freeze drying techniques offer advantages and are suitable for different applications and formulations. The choice of manufacturing method depends on factors such as the properties of the active ingredient, desired particle size distribution, and scalability of production.
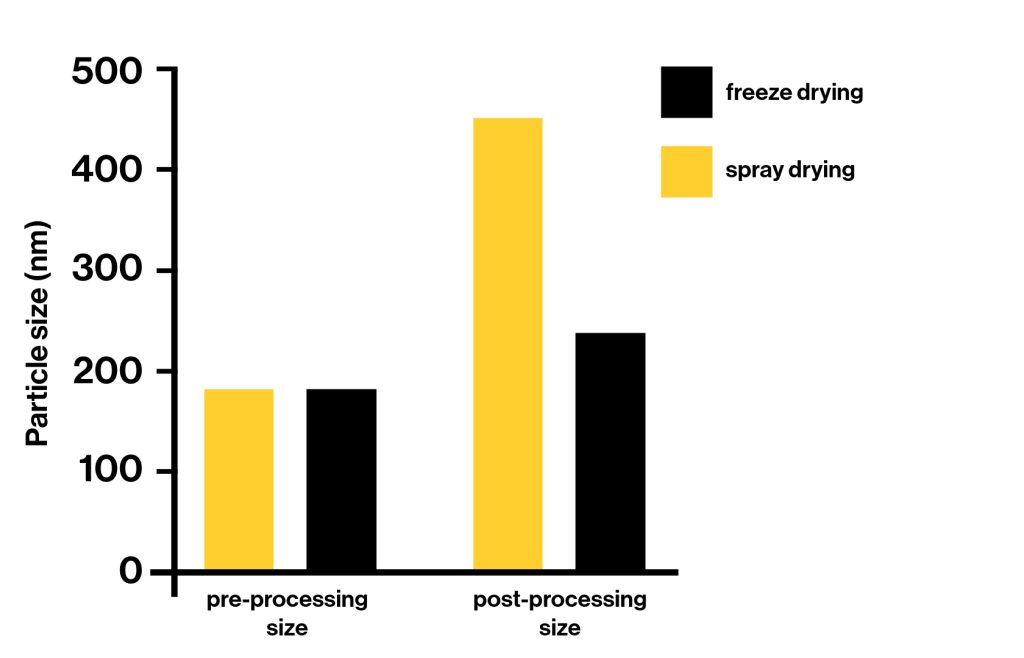
Vitamin C samples physicochemical properties comparison processed using freeze and spray drying
Physical Properties | Freeze Drying | Spray Drying |
Particle size after processing | 236nm | 451nm |
Moisture content | 2.36% | 5.8% |
Angle of repose | Excellent | Excellent |
Compressibility index | Excellent | Excellent |